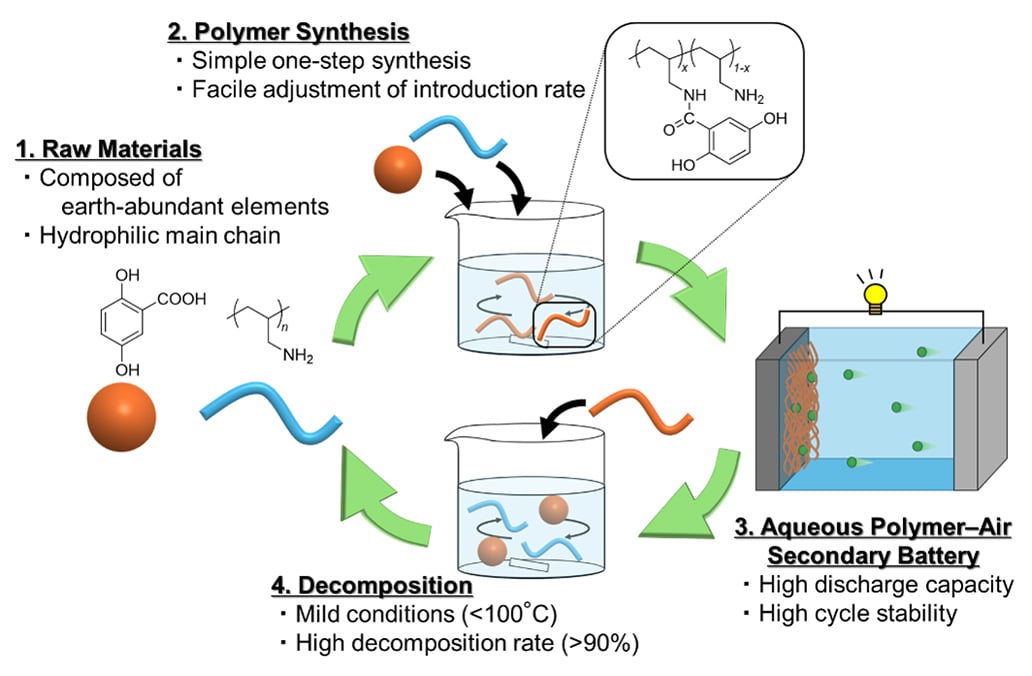
Aqueous batteries have been around for centuries. They are safe and relatively inexpensive, but their introduction to new energy storage systems such as grid storage and electric vehicles was limited. One main reason is the material compatibility: many electrode materials do not work well in aqueous electrolytes. Hydrophobicity was a barrier, especially for organic redox polymers. As with other polymer materials, they also face challenges when it comes to disassembly and recycling.
Now a research team from Tohoku University has developed a new organic redox polymer in cooperation with Nitto Boseki Co., Ltd., which deals with these long -term challenges.
In order to overcome the hurdles, the team P -Dihydroxybenzene – an organic molecule with high load storage capacity – introduced a polyamine that is water -soluble due to its positive load. This was achieved by a simple condensation reaction. The resulting polymer retains high hydrophilia, can be used as an electrodeactive material at room temperature (25 ° C) and divided into its raw components at temperatures below 100 ° C.
“This study offers a design strategy to make hydrophobic redox molecules compatible with aqueous systems,” said Kouki Oka, Associate Professor at the Institute for Multidisciplinary Research for Progressive Materials at the University of Tohoku. “With the combination of high -load storage capacities with recyclability, we can open up new directions for sustainable battery research.”
The results show two important advantages. First, the use of water -based electrolytes avoids the fire risk in connection with conventional combustible solvents. Second, since the new polymers are made from plenty of elements and can be easily decomposed, they can help reduce resource consumption and plastic pollution.
“Our next step is to evaluate the durability and other performance factors in order to understand the full potential of this material for real applications,” added Oka.
Research was published online in the Polymer Journal on August 26, 2025 and selected stars in Polymer Science 2025 for special expenses.